Glycan Analysis Services - Level 3
This module is an extension of the Level 1 and Level 2 N-glycan characterisation work to gain more detailed information on the individual N-glycan sites. This can be achieved by site occupancy analysis and detailed site-specific glycosylation analysis.
Level 3 N-glycan Site-Specific Glycosylation Analysis
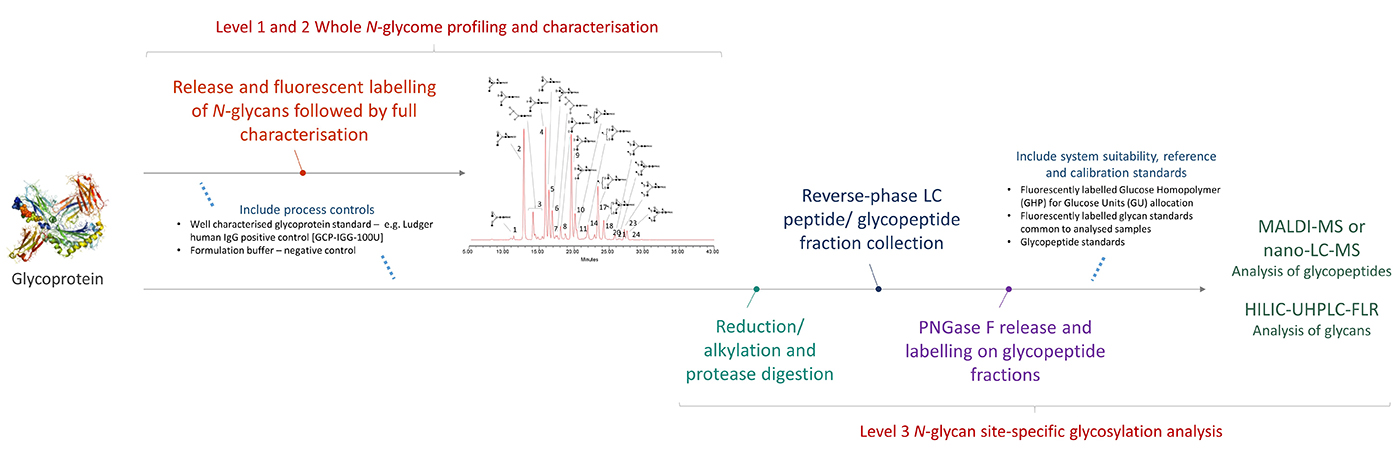
The number of potential N-glycosylation sites is determined by the amino acid sequence of the glycoprotein. This module provides HILIC-UPLC-FLR profiles for the N-glycans at each site occupied by N-glycans. The glycoprotein is digested into peptides and glycopeptides using proteases. These are then separated by C18 column chromatography and fractions collected. Each fraction is treated with PNGase F to release the N-glycans that are then fluorescently labelled and analysed by HILIC-UPLC-FLR to obtain an individual site glycosylation profile.
Determination of site glycosylation is a non-standard glycoprofiling module because the exact conditions required will differ for each glycoprotein analysed.
The amino acid sequence and the formulation buffer can both have an effect on the exact protocol followed as well as on generated data.
The analysis will be performed on a single glycoprotein sample, alongside Ludger positive and negative controls, and system suitability standards.
Glycan profiles for each individual site are identified by comparison of the data obtained from Level 1 and Level 2 analyses.
This module is suitable for:
- quality control - profile comparisons to monitor known structures
- monitoring batch to batch consistency
- comparability studies
In order to gain more detailed information on N-glycan % occupancy at each site, Level 3 site occupancy analysis is recommended.
Sample types:
Glycoprotein and glycopeptide samples composed of two or more N-glycosylation sites:
- Biopharmaceuticals: mAbs, glycoprotein hormones (e.g. follicle stimulating hormone (FSH) and erythropoietin (EPO), Fc fusion proteins, vaccines)
- Glycoproteins purified from cells and cell components
- Glycoproteins purified from biological fluids, tissues and others
- COVID-19 patient samples (e.g. plasma, tissues)
- SARS-CoV-2 infected cell lines
Workflow for Level 3 N-glycan Site-Specific Glycosylation Analysis
Report
Final report contains:
- HILIC-UPLC-FLR profiles for system suitability standards and Ludger positive and negative controls
- HILIC-UPLC-FLR glycan profiles of each isolated peptide and glycopeptide fraction and each isolated N-glycan site for client samples
- Glucose unit (GU) values assigned to glycan peaks
- Proposed glycan structures and their relative proportions at each site
- Summary of findings for each occupied N-glycan site (e.g. G0:G1:G2; % high mannose; % fucosylation)
