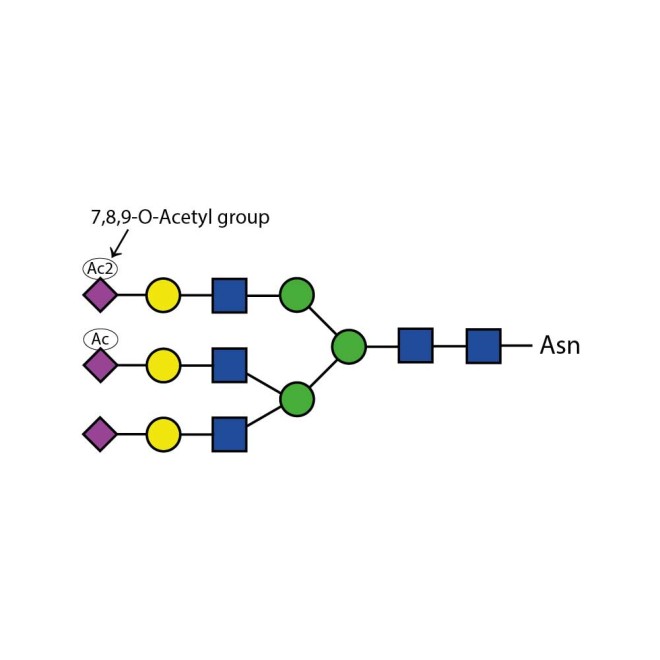
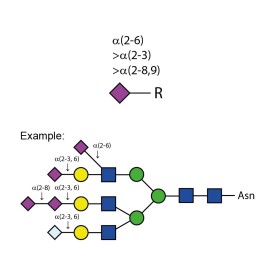
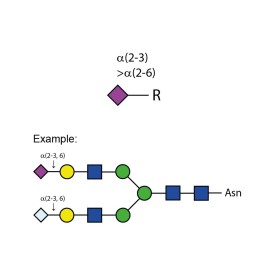
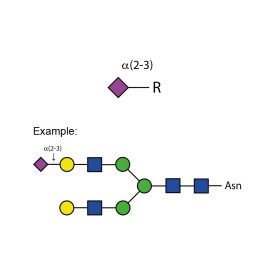
Sialate O-acetylesterase kit
To remove 9-, 8- and 7-O-acetyl groups from released sialic acids, released glycans or glycoproteins. Used for characterisation of highly sialylated biotherapeutics such as EPO, FSH and blood clotting factors. Kit includes enzyme plus reaction buffer. Sufficient for up to 50 samples.
Product specification
Contents:
Source: Recombinant Tannerella forsythia enzyme in E. coli
EC: 3.1.1.53
Alternate Names: sialate O-acetylesterase, N-acetylneuraminate acetyltransferase
LudgerZyme Acetyl Esterase (Supplied in PBS pH7.5 buffer containing 10 mM Tris-HCl)
LudgerZyme Acetyl Esterase RXN 10x buffer (500 mM sodium acetate pH5.5)
Number of Samples: Sufficient for up to 50 samples.
Amount of Sample: Up to 10 µg glycoprotein, up to 2.5 µg released glycans and up to 1 µg free sialic acid per digestion.
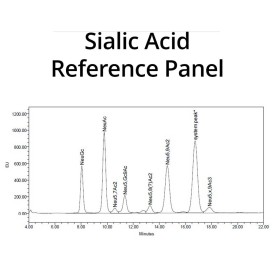
Suitable Samples: Acetyl esterase (sialate-O-acetylesterase) can act upon complex glycoprotein samples, such as erythropoietin (EPO), bovine submaxillary mucin and oral epithelial cell-bound glycans, and on N- and O-glycans released from a glycoprotein. Either fluorescently labelled or unlabelled glycans are suitable. It can also be used on released sialic acids.
Unit Definition: One unit (U) of acetyl esterase is defined as the amount of enzyme required to produce 300 µmole of 4-nitrophenol and acetate in 1 minute at 30°C in a buffer containing 50 mM Tris-HCl, 140 mM NaCl, pH 8.5, from 4-nitrophenyl acetate, a chromogenic esterase substrate.
Molecular Weight: 76.3 kDa.
Suggested usage:
1. To digest <10 µg of glycoprotein, make up a total reaction volume of 10 µL by dissolving the glycoprotein in 1 µL of LudgerZyme Acetyl Esterase RXN 10x buffer, 1 µL of LudgerZyme Acetyl Esterase and water to a final volume of 10 µL.
Use the same method for the digestion of <2.5 µg released glycans or <1 µg free sialic acid.
The reaction may be scaled up linearly to accommodate larger amounts of glycoprotein, released glycans or free sialic acids.
2. Incubate your samples in a water bath, oven or any other constant heating source at 37°C for 7 to 16 hours.
Note: Optimal incubation times and enzyme concentrations must be determined empirically for a particular substrate.
The samples are now ready for clean-up and subsequent analysis by HPLC.
Note: Optionally, the samples can be stored frozen at this point for analysis at a later date.
Storage: Store at 4°C. Protect from sources of heat and light. When stored correctly, the enzyme should be stable for 24 months from the date of purchase. Exposure to ambient temperatures (20 - 26°C) over 3 days does not result in a reduction of enzymatic activity.