Fetuin glycoprotein standard
The Fetuin Glycoprotein Standard is a highly sialylated glycoprotein standard which can be used as a positive control during glycan release and labelling (particularly for DMB sialic acid analysis). Fetuin exists in a variety of glycoforms containing bi-, tri-, and tetraantennary N-linked oligosaccharides with variable sialylation, as well as O-linked glycans.
1 vial containing 0.5 mg fetuin glycoprotein
Product Specification
The fetuin glycoprotein standard was developed for use during glycan release and labelling (particularly for DMB sialic acid analysis). Fetuin exists in a variety of glycoforms containing bi-, tri-, and tetra-antennary oligosaccharides with variable sialylation, as well as O-linked glycans.
Amount of Glycoprotein Supplied
GCP-FET-05 500 µg
GCP-FET-250U 250 µg
GCP-FET-50Ux4 50 µg per vial – 4 vials
Source Fetal calf serum
Form Dry. Lyophilised powder.
Molecular Weight 36 kDa (protein weight only)
Amount of Protein View the corresponding Certificate of Analysis for the range of each batch determined by BCA assay. (Value rounded to nearest μg).
Analysis
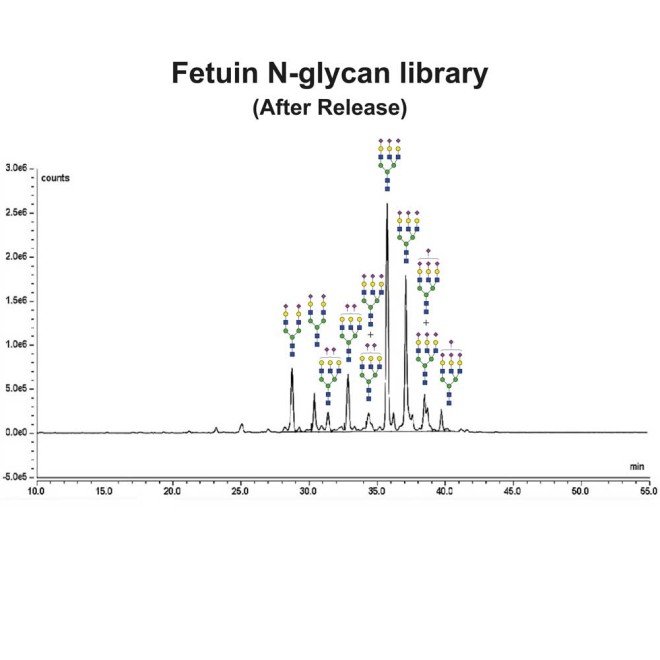
Fetuin glycans were released from the Fetuin Glycoprotein Standard (Cat# GCP-FET-05) using PNGase-F.
Following release, the glycans were labelled using 2-Aminobenzamide (2-AB) using the LudgerTag 2-AB Glycan Labelling Kit (Cat# LT-KAB-A2).
Figure 1 shows a LudgerSep N2 HPLC profile of bovine fetuin N-glycans. To thoroughly investigate the N-glycans, we first separate them based on charge on a LudgerSep C3 column (Figure 2) and then run each fraction on a LudgerSep N2 column.
Storage Refrigerate (-20°C) both before and after dissolving. This product is stable for at least 5 years as supplied.
Shipping The product is shipped at ambient temperature.
Handling Once dissolved, avoid repeated thawing and refreezing, storage over 3 hours at room temperature or above, exposure to light and long-term exposure to acid, as these will cause glycan desialylation.
Safety This product is non-hazardous and has been purified from natural sources, certified to be free of all hazardous material, including pathogenic biological agents.