LudgerZyme α(1-3,4) fucosidase kit
References:
1. H. Wu, O. Rebello, E.H. Crost, C. D. Owen, S. Walpole, C. Bennati-Granier, D. Ndeh, S. Monaco, T. Hicks, A. Colvile, P.A. Urbanowicz, M.A. Walsh, J. Angulo, D.I.R. Spencer, N. Juge. Fucosidases from the human gut symbiont Ruminococcus gnavus. Cell Mol Life Sci. 2021; 78(2): 675–693
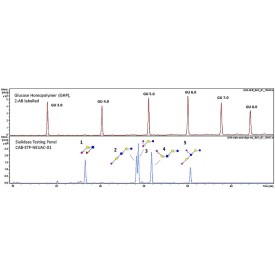
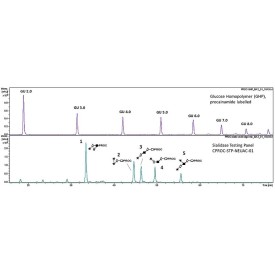
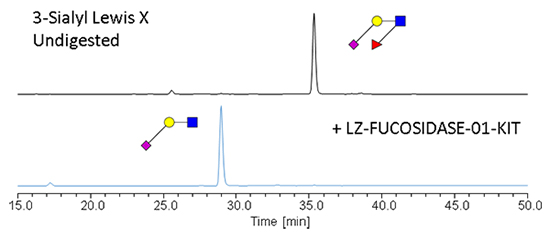
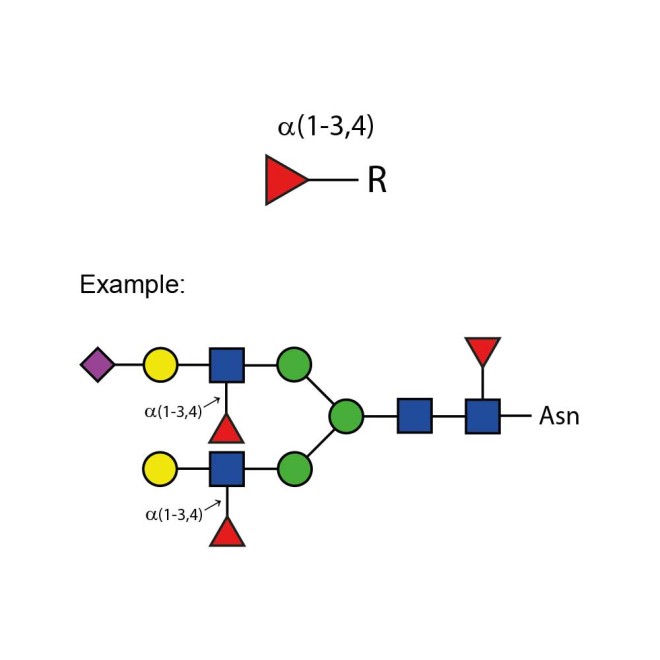
The enzyme recognises α1-3,4 fucosylated glycans (e.g. Lewis X/A epitopes, including their sialylated counterparts) and hydrolyses terminal α1-3 and α1-4 fucosyl linkages in these substrates without the need to remove sialic acid. Due to this proprietary feature, this enzyme can be used to streamline your workflow, making it both cost and time-efficient as there is no need to use sialidase enzyme before targeting digestion of antennary fucosylation.
Another advantage is that the enzyme can be used for monitoring α1-3,4 fucosylation on free glycans as well as glycoproteins1 which eliminates the need for chemical or enzymatic release of glycans from glycoproteins.
Product Details:
- Part Number: LZ-FUCOSIDASE-01-KIT
- Amount of Enzyme: 50 µL
- Non-sialidase dependant hydrolysis of antennary fucose moieties
- Effective on both glycopeptides and free glycans
- Highly specific (α1-3,4 fucosylated glycans)
- Kit includes enzyme plus reaction buffer.
- Sufficient for up to 50 samples.
Product specification
α1-3,4 fucosidase is a broad specificity recombinant protein from Ruminococcus gnavus, E1 strain, expressed in Escherichia coli 1. The enzyme recognises α1-3,4 fucosylated glycans (e.g. Lewis X/A epitopes, including their sialylated counterparts) and hydrolyses terminal α1-3 and α1-4 fucosyl linkages in these substrates without the need to remove sialic acid. It can be used for monitoring α1-3,4 fucosylation on free glycans and glycoproteins1.
Application:This fucosidase can be used to remove α1-3 and α1-4 linked fucose from free glycans and glycoproteins.
Source: Recombinant protein from Ruminococcus gnavus E1 strain, expressed in Escherichia coli
EC: 3.2.1.51
Alternate Names: α-L-fucoside fucohydrolase, α-L-fucosidase, α-(1-3,4) fucosidase
Contents:

LudgerZyme α1-3,4 Fucosidase (Supplied in 200 mM citrate buffer pH 6 containing 250 mM NaCl)
LudgerZyme α1-3,4 Fucosidase 5x Reaction Buffer (250 mM sodium phosphate pH 6)
Number of Samples: Sufficient for up to 50 samples.
Amount of Sample: 50 µl.
Suitable Samples: α1-3,4 fucosidase can act upon oligosaccharides, complex glycans and glycoprotein samples, such as those displaying (sialylated) Lewis X/A epitopes. Either fluorescently labelled or unlabelled glycans are suitable.
pH Optimum: 6.0. Working pH range is between 5.5 and 7, at which the enzyme maintains at least 50% of its maximum activity.
Molecular Weight: 63.5 kDa.
Suggested Protocol:
- Add up to 15 µg of glycoprotein or 0.5 µg of free oligosaccharide/ glycan to reaction tube. Free glycans can be native or fluorescently labelled.
- Add pure water to a total of 7 µl.
- Add 2 µl of 5x Reaction Buffer.
- Add 1 µl of α1-3,4 Fucosidase. Vortex sample and briefly centrifuge.
- Incubate at 37°C for 1 hour.
The reaction may be scaled-up linearly to accommodate larger amounts of glycoprotein, released glycans or oligosaccharides. Free native and fluorescently labelled glycans and oligosaccharides can be cleaned-up using Post-Exoglycosidase Clean-up Spin Columns [LC-EXO-A6] or Post-Exoglycosidase Clean-up 96-well Plate [LC-EXO-96].
Storage: Store at 4°C. Do not freeze. Protect from sources of heat and light. When stored correctly, the enzyme should be stable for 12 months from date of purchase. Exposure to ambient temperatures over several days does not result in a reduction of enzymatic activity.
License: LZ-FUCOSIDASE-01-KIT is manufactured under licence from the Quadram Institute Bioscience (Norwich, UK).


 Product guide
Product guide