Glycoprotein & Glycopeptide
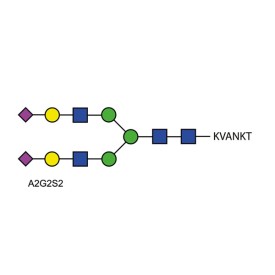
A2G2S2 quantitative glycopeptide standard
BQ-GPEP-A2G2S2-10U
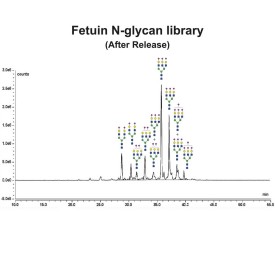
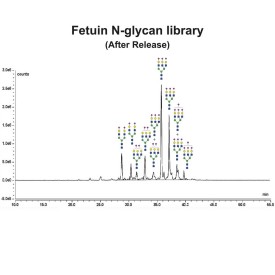
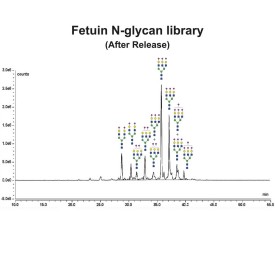
Fetuin glycoprotein standard
GCP-FET-05
Fetuin glycoprotein standard
GCP-FET-250U
Fetuin glycoprotein standard
GCP-FET-50U-X4
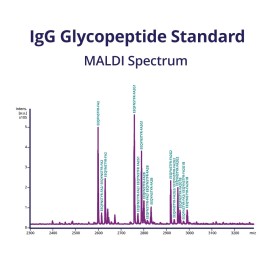
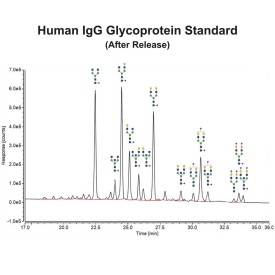
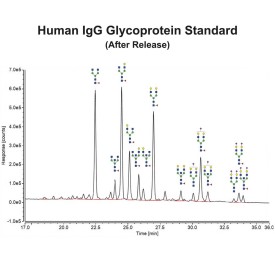
Human IgG Glycoprotein Standard
GCP-IGG-100U
Human IgG Glycoprotein Standard
GCP-IGG-50U
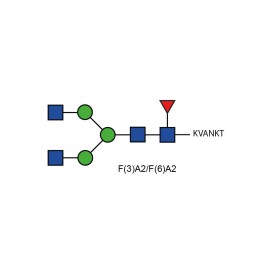
GPEP FA2 glycopeptide standard
GPEP-FA2-01
GPEP IGG glycopeptide standard
GPEP-IGG-01
Showing 1 to 8 of 8 (1 Pages)


-275x275.jpg)





-275x275.jpg)