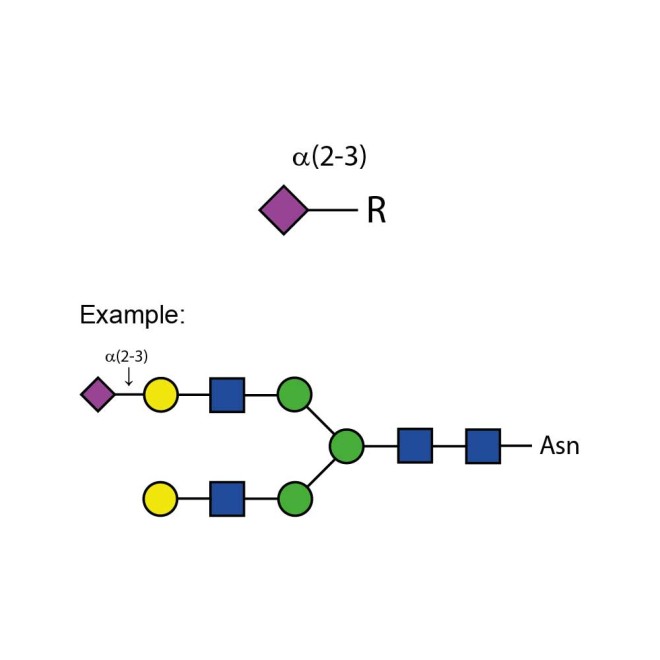
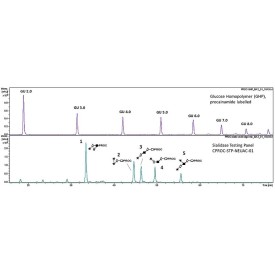
Sialidase Sp α-(2-3)
References:
1. Corfield, A. P., H. Higa, J. C. Paulson and R. Schauer. The specificity of viral and bacterial sialidases for alpha(2-3) and alpha(2-6)-linked sialic acids in glycoproteins. Biochim Biophys Acta 744: 121-12 6 (1983).
2. Dwek, R. A., C. J. Edge, D. J. Harvey, M. R. Wormald and R. B. Parekh. Analysis of glycoprotein-associated oligosaccharides. Ann Rev Biochem 62: 65-100 (1993).
3. Kobata, A. Use of endo- and exoglycosidases for structural studies of glycoconjugates. Anal Biochem 100: 1-14 (1979).
4. Ohta, Y., Y. Tsukada and T. Sugimori. Purification and properties of neuraminidase isoenzymes in Arthrobacter ureafaciens mutant. J Biochem (Tokyo) 106: 1086- 1089 (1989).
5. Prime, S., J. Dearnley , A. M. Venton, R. B. Parekh and C. J. Edge. Oligosaccharide sequencing based on exo- and endoglycosidase digestion and liquid chromatographic analysis of the products. J Chromatogr A 720: 263-274 (1996).
6. Uchida, Y., Y. Tsukada and T. Sugimori. Enzymatic properties of neuraminidases from Arthrobacter ureafaciens. J Biochem (Tokyo) 86: 573-58 5 (1979).
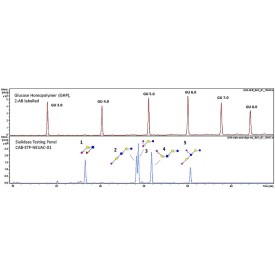
Sialidase Sp cleaves the non-reducing terminal α(2-3) unbranched sialic acid residues from complex
Product specification
Contents:
Source: Recombinant Streptococcus pneumoniae in E. coli
EC: 3.2.1.18
Alternate Names: Neuraminidase, N-acetylneuraminate glycohydrolase, Exo-α-sialidase
Sialidase Sp in 50 mM sodium phosphate, pH 7.5
5x Reaction Buffer 250 mM sodium phosphate, pH 6.0
Specific Activity: = 250 U/mg
Activity: = 10 U/mL
Molecular weight: ~75,000 daltons
pH optimum 6.0
Suggested usage: 1. Add up to 100 µg of glycoprotein or 1 nmol of oligosaccharide to tube.
2. Add water to 14 µL
3. Add 4 µL 5X Reaction Buffer.
4. Add 2 µL α(2-3) Sialidase Sp.
5. Incubate at 37°C for 1 hour
Desialylation may be monitored by SDS-PAGE if the size differential between native and desialylated protein is sufficient for detection.
Specificity: Cleaves the non-reducing terminal α(2-3) unbranched sialic acid residues from complex carbohydrates and glycoproteins.
Specific Activity: Defined as the amount of enzyme required to produce 1 µmole of methylumbelliferone in 1 minute at 37°C, pH 5.0 from MU-NANA [2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid].
Storage Store enzyme at 4°C.
Desialylation may be monitored by SDS-PAGE if the size differential between native and desialylated protein is sufficient for detection.
Applications
- Structural analysis of oligosaccharides
- Determining sialic acid linkage
- Glycoprotein deglycosylation
- Removing heterogeneity from glycoproteins
Recommended Reagents
included with 20 µL and 60 µL pack sizes:
1 vial: Reaction buffer – 400 µl
250mM Sodium phosphate, pH 6.0
Formulation
The enzyme is provided as a sterile-filtered solution in in 50 mM Sodium phosphate pH 7.5.
Stability
Stable at least 12 months when stored properly. Several days exposure to ambient temperatures will not reduce activity